CATALOGING NATURAL SIALIC ACIDS AND OTHER NONULOSONIC ACIDS (NULOS), AND THEIR REPRESENTATION USING THE SYMBOL NOMENCLATURE FOR GLYCANS (SNFG)
1Glycobiology Research and Training Center, University of California, San Diego, CA, USA
2Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Moscow, Russia
3National Center for Biotechnology Information, National Library of Medicine, NIH, Bethesda, MD, USA
4Department of Chemistry, University of California, Davis, CA, USA
5Biognos AB, Göteborg, Sweden
6Institute of Veterinary Physiology and Biochemistry, Justus-Liebig-University Giessen, Giessen, Germany
7Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Moscow, Russia
8Human Health Therapeutics Research Centre, National Research Council of Canada, Ottawa, Ontario, Canada
9Glycobiology Research and Training Center, University of California, San Diego, CA, USA
10Human Health Therapeutics Research Centre, National Research Council of Canada, Ottawa, Ontario, Canada
11Complex Carbohydrate Research Center, University of Georgia, Athens, GA, USA
12Department of Biological Chemistry, Johns Hopkins University School of Medicine, Baltimore, MD, USA
13National Center for Biotechnology Information, National Library of Medicine, NIH, Bethesda, MD, USA
14Bijvoet Center, Utrecht University, Utrecht, The Netherlands
15State University of New York, Buffalo, NY, USA
|
16Other members of the SNFG Discussion group: Alan Darvill, University of Georgia, USA Anne Dell, Imperial College London, UK Bernard Henrissat, Technical University of Denmark, Denmark Carolyn Bertozzi, Stanford University, USA Frederique Lisacek, Swiss Institute of Bioinformatic, Switzerland Gerald Hart, University of Georgia, USA Hisashi Narimatsu, Research Center of Medical Glycoscience, Japan Hudson Freeze, Sanford-Burnham-Prebys Research Institute, USA Issaku Yamada, The Noguchi Institute, Japan James Paulson, Scripps Research Institute, USA Jamey Marth, University of California Santa Barbara, USA JFG Vliegenthart, Bijvoet Center, The Netherlands Kiyoko F. Aoki-Kinoshita, Soka University, Japan Marilynn Etzler, UC Davis, USA Markus Aebi, ETH Zürich, Switzerland Matthew Campbell, Institute for Glycomics, Griffith University, Australia Michael Tiemeyer, University of Georgia, Complex Carbohydrate Research Center, USA | Minoru Kanehisa, Kyoto University, Japan Naoyuki Taniguchi, Riken Global Research Cluster, Japan Nathan Edwards, Georgetown University, USA Nicolle Packer, Macquarie University, Australia Pamela Stanley, Albert Einstein dicine, USA Pauline Rudd, National Institute for Bioprocessing Research & Training, UK Peter Seeberger, Max-Planck-Institute of Colloids and Interfaces, Germany Raja Mazumder, The George Washington University, USA Rene Ranzinger, University of Georgia, USA Richard Cummings, Harvard Medical School, USA Roger Sayle, NextMove Software, UK Ronald Schnaar, Johns Hopkins University School of Medicine, USA Serge Perez, French National Centre for Scientific Research, France Stuart Kornfeld, Washington University in St. Louis, USA Taroh Kinoshita, Osaka University, Japan William York, University of Georgia, USA |
KEYWORDS: nonulosonic acid, NulO, sialic acid, symbol nomenclature, glycans
Glycobiology, 2023, ò. 33(2), ñòð. 99-103
DOI: 10.1093/glycob/cwac072 , PMID: 36648443
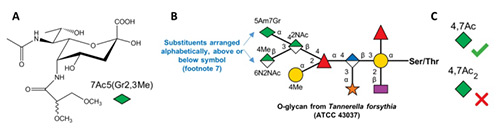
Nonulosonic acids or non-2-ulosonic acids (NulOs) are an ancient family of 2-ketoaldonic acids (α-ketoaldonic acids) with a 9-carbon backbone. In nature, these monosaccharides occur either in a 3-deoxy form (referred to as ‘sialic acids’) or in a 3,9-dideoxy ‘sialic-acid-like’ form. The former sialic acids are most common in the deuterostome lineage, including vertebrates, and mimicked by some of their pathogens. The latter sialic-acid-like molecules are found in bacteria and archaea. NulOs are often prominently positioned at the outermost tips of cell surface glycans, and have many key roles in evolution, biology and disease. The diversity of stereochemistry and structural modifications among the NulOs contributes to more than 90 sialic acid forms and 50 sialic-acid-like variants described thus far in nature. This paper reports the curation of these diverse naturally occurring NulOs at the NCBI sialic acid page (www.ncbi.nlm.nih.gov/glycans/sialic.html) as part of the NCBI-Glycans initiative. This includes external links to relevant Carbohydrate Structure Databases. As the amino and hydroxyl groups of these monosaccharides are extensively derivatized by various substituents in nature, the Symbol Nomenclature For Glycans (SNFG) rules have been expanded to represent this natural diversity. These developments help illustrate the natural diversity of sialic acids and related NulOs, and enable their systematic representation in publications and online resources.