Navigation:
Course program
Supplementary material
Lection illustrations
Video and verbatim
Control tests
Course program
(N - links to illustrations, in Russian)

Chapter 1. Intro
- Hostory of NMR. What is included in this course and why.01
- NMR spectroscopy in organic chemistry. Advantages, limitations and unique opportunities of this method. The price of NMR-spectroscopic investigation.1
- Information provided by NMR spectrum: quantity of signals, their position, shape and square. Examples of the simplest spectra. Spectrum integration.2
- Physical basis of the NMR phenomenon. Nuclei in magnetic field and their shielding by electrons and chemical surrounding. Resonance transitions, gyromagnetic ratio.3
- Chemical shift and its measurement units. Physical basics and idea of continuous-wave NMR experiment. What is the NMR spectrum?3
- Chemically important magnetic nuclei. Absolute and relative sensitivity. Standards of chemical shifts, TMS/DSS, chemical shift ranges for different nuclei.5
- Proton chemical shifts. Rough dependence on the neighboring atoms and bonds. Characteristic values for different structural fragments.4
- 13C chemical shifts. Rough dependence on the neighboring atoms and bonds. Characteristic values for different structural fragments. Additive schemes of chemical shift simulation.6
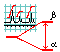
Chapter 2. Spin coupling
- The reasons for multiplicity. Spin coupling with several identical neighbors. Pascal triangle. The invariance of signal shape on the splitting order.7
- Other examples of spin coupling. Various cases with three neighbors. Degeneration of a DD signal into a triplet.8
- Molecular symmetry, chemical and magnetic equivalence of atoms.10
- Spin coupling with nuclei with spin of 1. The isotope shift. The spectrum of real DMSO-d6.9
- Basic proton-to-proton coupling constants.11
- Spectra of 1,2,4-dibrombenzene and β-D-galactopyranose recorded on spectrometers with different frequency.12
- Examples of spin coupling: proton spectra of substituted aromatics. Minor lines in the spectra of molecules with several chemically equivalent nuclei.131415
- Examples of spin coupling: spectra perturbations when coupling constants in substituted aromatics are not exactly equal each to other.16
- Examples of spin coupling: the theoretical proton spectrum of allyl bromide.17
- Heteronuclear spin coupling. Dependence of coupling constants on the gyromagnetic ratio. The idea of broadband decoupling. 13C Gated experiment, its advantages and disadvantages.29
- 13C satellites. Disappearance of proton equivalence in spectra of their satellites. Spin coupling to quadrupole nuclei.31
- Basic proton-to-carbon coupling constants. Dependence on the hybridization state. The sign of coupling constants.30

Chapter 3. Structure-to-spectrum correlation
- Roof effect. Degeneration of two doublets into a singlet. Variants of roof effect in the ABC spin system.18
- Examples of spectra. Aliphatic and aromatic protons. Signal overlap. Defects in line shape, signal square and roof effect.19-25
- Proton spectra assignment using multiplicity analysis and exact coupling constant measurement. 19-25
- NMR spectra of mixtures. Separation of such spectra into subspectra of ingredients. Quantitative analysis of mixtures.2627
- Coupling constant to structure correlation. Dependence of heminal constants on the valent angle, neighboring π-electrons and substituents. Dependence of vicinal constants on the torsion angle and bond length. Carplus equation.32
- NMR spectra of the first and non-first order. Theoretical and experimental spectra of highly coupled systems.35
- Temperature variations of the NMR spectra. Intra- and intermolecular exchange. Dynamic effects on spin coupling and signal position. The energetic barrier of transition. Signals of labile protons.33
- Typical energies of widespread processes of chemical and conformational exchange. NMR response time.34
- Dependance of NMR observables on two-centered H-bonding. Signal displacement for bridge hydrogen, hydrogen donor and acceptor. Coupling to and through bridge hydrogen. 36
- Elucidation of structure from 1D 1H spectra and brutto-formula.
- Elucidation of structure from a set of 2D spectra: COSY, TOCSY, HSQC, HMBC. 5657758384
- An example of structural problem solved by COSY, HSQC and NOESY. 89909192
- NMR spectra simulation. Empirical, semi-empirical and quantum-mechanical approaches. HOSE. Usage of artificial neural networks.93
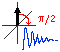
Chapter 4. Pulse NMR spectroscopy
- The classical and pulse NMR. Limitations of continuous wave NMR. Short and long pulses. "Bell tuning".37
- FID and NMR spectrum. Fourier transformation. Lorenz line. NMR data in frequency and time domains.38
- The scheme of elementary NMR experiment. Signal/noise ratio and FID accumulation. Basic parameters of 1D NMR experiment. Relaxation delay, acquisition time. Natural and digital resolution. The spectrum window and the number of data points.40
- The nucleus magnetic moment and the vector of macroscopic magnetization (MM). The oscillating radio-frequency field representation by precessing magnetic moments. Rotating co-ordinate frame.43
- Post-pulse MM evolution in rotating frame. Pulse types.43
- What happens if transition is not exactly in resonance with irradiation? Detecting several signals at once, blurring the MM vector into precessing components in rotating frame. Affect of pulses on the population of spin states.45
- Chemical shift and coupling constants consideration within a formalism regarding vectors in rotating frame. What do FID oscillations really represent?45
- Longitudinal and transversal relaxation. Exponential decay of free induction. Inversion-recovery method.46
- Spin echo and magnetization component refocusing. Differences between homo- and heteronuclear spin coupling. Broad band decoupling in proton spectra.47
- Advantages of polarization transfer. Selective and non-selective polarization transfer. Dependence of the PT efficiency on gyromagnetic ratios of irradiated and observed nuclei.48
- Cross-relaxation and Nuclear Overhauser Effect (NOE). Structural dependencies of sign and absolute value of NOE. Investigation of proton spatial contacts with NOE spectroscopy.61

Chapter 5. Various NMR experiments
- Two-dimensional correlation spectroscopy. Coherence transfer. COSY experiment and its pulse diagram. 2D Fourier transformation.5051
- Homo- and heteronuclear COSY and structural information provided by these experiments. Proton spectrum assignment using COSY data.52-57
- Selective suppression of spin coupling. 1D spectra of double resonance. Partial spin decoupling. The Bloch-Ziegert shift. Examples of structural investigations with a series of double resonance experiments.585960
- NOESY and ROESY experiments. Signal phase in two-dimensional NOE spectra. Dependence on the magnetic field intensity.6265
- Difference mode experiments. Positioning the substituents and proton spectrum assignment by difference mode NOE spectroscopy. Examples of dibromotoluene and substituted porphyrine.6364
- Non-correlational NMR experiments and information they provide. A plan of structural investigation with NMR spectroscopy. 70
- Pseudo-2D NMR experiments: diffusion ordered (DOSY) and coupling constant ordered (J-resolved) spectroscopy. 7172
- Opportunities of APT, INEPT, DEPT and H-Gated experiments. Polarization transfer and sensitivity. Carbon spectra editing.49
- Homonuclear spin correlations. Types of COSY: first pulse length variation, coherence transfer, double-quantum filter. TOCSY experiment. 73747576
- Heteronuclear spin correlations. HETCOR, HSQC, and HMQC experiments. Coherence transfer, editing, and proton decoupling in HSQC.808182
- Comparison of HSQC and HMBC and information provided by one- and two-dimensional versions of these experiments. 8384
- Combined correlations (HSQC-TOCSY, HSQC-NOESY). 85
- Direct non-proton homonulear correlation (INADEQUATE).77
- Spatial correlations and information they provide. Magnetization transfer during NOE observation and its usage in binding studies. STD, WaterLOGSY, ILOE and INPHARMA experiments. Dependence of relayed magenization on molecular relaxation and mixing time.7879
- 3D NMR experiments ({H,C,N} and {H,H,N}) used in proteomics. X-edited and X-filtered experiments. 868788

Chapter 6. Practical realization of NMR
- Pre-FT FID processing. Weight functions, Lorenz and Gauss line. Exponential multiplication and Gauss enhancement. Zero filling, truncation and apodization.39
- Analog signal and its digital representation. Digitizing the NMR signal. Receiver gain.41
- The principal scheme of the NMR spectrometer, its control computer and I/O devices. The supercon and how it works. NMR probe heads: types, applications.4142
- The data excerption rate required, demands to memory volume and bit depth. Niquist criterion. Reflected signals and their phase. The band filter application.41
- The real and imaginary parts of Fourier image. Signal phase. Quadrature detection. The idea of phase cycles.44
- What are resolution and sensitivity? Resonance condition stabilization in time and space. Gradient shims. The deuterium stabilization (LOCK). The resolution criteria: reference LOCK level, FID square, line form. Typical mistakes in shimming.66
- Selection of a solvent and sample preparation. The affect of NMR tube, sample volume, concentration and viscosity. The properties of widespread NMR solvents.6768
- Quantitative measurements of signal square and NOEs.69
- Redundant peak suppression in 1D- and 2D-NMR spectroscopy. Decoupler and its hardware implementation.
- Widespread NMR processing software: MestreNova, ACDLabs, XWinNMR.
Supplementary
Illustration to lectures (inscriptions in Russian)
Monographs:
 A. Derome "Modern NMR spectroscopy in chemical research", Moscow, Mir, 1992 (DjVu, 4.7 Mb, in Russian)
A. Derome "Modern NMR spectroscopy in chemical research", Moscow, Mir, 1992 (DjVu, 4.7 Mb, in Russian)
 T. Claridge "High-resolution NMR techniques in organic chemistry", Pergamon, 1999 (PDF, 6.0 Mb, in Russian)
T. Claridge "High-resolution NMR techniques in organic chemistry", Pergamon, 1999 (PDF, 6.0 Mb, in Russian)
 K. Denk "NMR spectroscopy", University of Guelph, 2005 (PDF, 4.6 Mb)
K. Denk "NMR spectroscopy", University of Guelph, 2005 (PDF, 4.6 Mb)
 E. Becker "High resolution NMR: theory and chemical applications", Academic Press, 2000 (PDF, 17.5 Mb)
E. Becker "High resolution NMR: theory and chemical applications", Academic Press, 2000 (PDF, 17.5 Mb)
 J. Lambert, E. Mazzola "Nuclear magnetic resonance spectroscopy", Pearson Education Inc., 200x (PDF, 18.8 Mb)
J. Lambert, E. Mazzola "Nuclear magnetic resonance spectroscopy", Pearson Education Inc., 200x (PDF, 18.8 Mb)
 A. Rahman, M. Choudhary "Solving problems with NMR spectroscopy", Karachi, 200x (PDF, 17.1 Mb)
A. Rahman, M. Choudhary "Solving problems with NMR spectroscopy", Karachi, 200x (PDF, 17.1 Mb)
 H. Günther "NMR spectroscopy. An introduction", Moscow, Mir, 1984 (PDF, 8.6 Mb, in Russian)
H. Günther "NMR spectroscopy. An introduction", Moscow, Mir, 1984 (PDF, 8.6 Mb, in Russian)
Workbooks (Ph. Toukach):
 Application of 2D NMR spectroscopy to organic chemistry - three examples of structural investigation (PDF, 4.1 Mb, in Russian)
Application of 2D NMR spectroscopy to organic chemistry - three examples of structural investigation (PDF, 4.1 Mb, in Russian)
 Modern NMR spectroscopy in structural studies of natural carbohydrates - lection slides
Modern NMR spectroscopy in structural studies of natural carbohydrates - lection slides
 Model study of a tetrasaccharide repeating unit by 2D NMR - spectra and description (PDF, 0.9 Mb)
Model study of a tetrasaccharide repeating unit by 2D NMR - spectra and description (PDF, 0.9 Mb)
 Application of 2D NMR spectroscopy to carbohydrate research - an example of natural structure elucidation (PDF, 1.3 Mb, in Russian)
Application of 2D NMR spectroscopy to carbohydrate research - an example of natural structure elucidation (PDF, 1.3 Mb, in Russian)
 Illustrations to lectures in one file (PDF, 9.9 Mb)
Illustrations to lectures in one file (PDF, 9.9 Mb)
 Introduction to pulse NMR (ZIPped DOC, 1.3 Mb, in Russian)
Introduction to pulse NMR (ZIPped DOC, 1.3 Mb, in Russian)
 NMR in the research of natural carbohydrates (ZIPped DOC, 0.3 Mb, in Russian)
NMR in the research of natural carbohydrates (ZIPped DOC, 0.3 Mb, in Russian)
 Monosaccharide nomenclature (SK2, ACDLabs ChemSketch, 108 Kb)
Monosaccharide nomenclature (SK2, ACDLabs ChemSketch, 108 Kb)
Video & verbatim
 Download lections: verbatim + slides + comments (PDF, 21 Mb, 247 p., in Russian)
Download lections: verbatim + slides + comments (PDF, 21 Mb, 247 p., in Russian)
Load lection [in Russian]:
1
2
3
4
5
6
7
Control tests and questions
 |
Download tests for self-control - spectra assignment, structure elucidation using 2D spectra, structure elucidation using 1D spectrum and a brutto-formula (ZIP archive with 18 TIFF images, 1.1 Mb) |
Structure - 1D spectrum correlation
- Which characteristic of the NMR spectrum corresponds to a number of atoms that produced a signal?
- Which characteristic of the NMR signals best suits the distribution of the electronic density over a molecule?
- What are chemical shifts measured in?
- What are coupling constants measured in?
- Which atoms are called "magnetically equivalent"?
- What are the limitations of the Pascal triangle application?
- Which signal corresponds to a proton with two identical closely-located neighbors and one distant neighbor?
- Which signal corresponds to a proton with three identical distant neighbors and one closely-located neighbor?
- What will a proton signal look like in a spectrum of H2N-ÑÎ-ÑÍD2?
- What will an emphasized proton signal look like in a spectrum of Br2ÍÑ-ÑÍD-COOH?
- What is an "isotope shift"?
- What will proton signals look like in a spectrum of 3-bromotoluene if all meta-couplings are equal and all ortho-couplings are equal?
- In which circumstances "roof" in a system of two protons is steeper?
- Which peculiarities of a molecule usually lead to non-first-order spectra?
- What are the main approaches to solve a problem of signal overlapping?
Chemical shifts and coupling constants
- What are chemical shifts referenced to?
- How does atom hybridization state affect its chemical shift?
- How is chemical shift affected by the electronegativity of neighboring atoms?
- What are the typical values of chemical shifts in aromatics?
- What are the typical values of coupling constants through various number of bonds in a benzene ring?
- What is a characteristic value of the heminal coupling constant in a substituted ethylene?
- What are the typical values of coupling constants through three bonds in aliphatics?
- Why coupling constants through four bonds are often observed in aromatics, but seldom observed in aliphatics?
- How will an absolute value of a heminal coupling constant change with increase of the central atom valent angle from 105º äto117º?
- How will a value of a vicinal coupling constant change with increase of the torsion angle from 0º to 180º?
- How are vicinal and heminal coupling constants affected by electronegative substituents?
- How are vicinal coupling constants affected by the bond length of the central bond?
- Which NMR observables depend on H-bonding?
- How do chemical shifts of hydrogen donor and acceptor depend on the energy of the two-centered H-bond?
NMR as a physical phenomenon
- What does the energetic gap between nuclear spin states depend on?
- What does the magnetic resonance frequency of a certain nucleus depend on?
- Which nuclei valuable in chemistry of natural compounds are magnetically active and have a spin of 1/2?
- What are the peculiarities of NMR spectroscopy of the quadrupole nuclei?
- What is the difference between absolute and relative NMR sensitivity?
- What is a gyromagnetic ratio?
- Which spin systems produce NMR spectra that require quantum-mechanical formalism even for qualitative prediction?
- What is the difference between transversal and longitudinal relaxation?
- Which type of relaxation is always faster than another type?
- How does faster relaxation affect the NMR signals?
- What is the origin of 13Ñ satellites in proton spectra?
- How one can control the effects introduced to NMR spectra by the presence of quadrupole nuclei?
Digital processing of the NMR data
- What is the difference between analog and digital signals?
- What are the amplitude, frequency and phase of a signal?
- What is a digital resolution and what are its differences from the natural one?
- How is an NMR spectrum affected by the multiplication of FID by an exponent?
- How is an NMR spectrum affected by the multiplication of FID by Gauss function?
- What are truncation and apodization of FID designed for?
- How can FID be obtained from an NMR spectrum?
- How can a time-resolved pulse-induced electromagnetic response be converted to the frequency spectrum?
- Which factors affect the accuracy of spectrum integration?
- How the molecular flexibility affects the NMR simulation accuracy?
- Which nuclei are subject to efficient NMR simulation by incremental schemes?
- How can HOSE approach be applied to the NMR spectra simulation?
- How can artificial neural networks be applied to the NMR spectra simulation?
- What is the minimal theory level and a basis set suitable for GIAO NMR simulation?
Practical implementation of NMR
- How do the NMR spectra depend on the spectrometer frequency?
- In which device does the electromagnetic irradiation of a sample happen?
- Which device acquires the sample response to pulse irradiation?
- Which acquisition parameters affect signal-to-noise ratio?
- What are interrelationships between acquisition time, spectrum width and number of data points in a FID signal?
- Which criteria should data excerption rate fit to avoid reflected signals?
- Which device allows the millionfold decrease of demands to ADC speed?
- How are the reflected NMR signals suppressed?
- What is quadrature detection designed for?
- What is a purpose of gradient coils?
- Which field gradients exhibit considerable drift from sample to sample?
- What is a purpose of LOCK?
- What are the main criteria to estimate the resolution tuning quality?
- Why is a sample spinned around 0z axis?
- What is a typical line half-width in routine experiments in DMSO-d6?
- What is a phase cycle?
- How does a spectrum depend on the adequate setting of the receiver gain?
- What are the main approaches to solve a problem of insufficient sensitivity?
- What are the main approaches to solve a problem of insufficient resolution?
- What are temperature limits for typical NMR experiments?
Sample preparation
- What is an optimal solvent volume and head of sample in 5 mm sample tube?
- What are the advantages and disadvantages of DMSO-d6 as an NMR solvent?
- What are the advantages and disadvantages of CDCl3 as an NMR solvent?
- What can be deduced from the observation of gradient of non-symmetrical line shape deviations?
- What are the advantages and disadvantages of 5 mm sample tubes as compared to 10 mm tubes?
- How is the NMR spectrum affected by the sample viscosity?
- How much compound is needed to obtain a 13Ñ NMR spectrum in several hours?
- What are Shigemi tubes and why are they needed?
- What are advantages and disadvantages of the external reference as compared to the internal one?
- How would you solve a problem of masking the signals by a signal of water?
Dynamics
- Why are –NH2 and –ÎÍ signals often broadened?
- What happens to an NMR spectrum when you heat a sample containing atoms non-equivalent in different conformations only?
- What happens to an NMR spectrum when you add water to a sample having labile protons?
- What is the characteristic NMR time scale?
- What is a coalescence temperature?
- How can a barrier of transition between two molecular forms be measured using NMR spectroscopy?
- Why is a proton spectrum of ethanol independent on the molecule conformation?
- Why does the acidization of ethanol remove coupling with hydroxyl group?
- How can one compute an average chemical shift of signals of two proton states?
- Why are the spectra of saccharides in D2O much simpler than in CDCl3?
Pulse NMR
- Which properties of the NMR spectrum are affected by the pulse length and how?
- How does the pulse length correlate with its power?
- What is a purpose of selective pulses?
- Why do we need a delay between the pulse and acquisition?
- Why do we need a delay between scans?
- Which properties of the NMR spectrum are affected by free induction decay speed, and what does this speed depend on?
- What does the rotating frame revolution rate depend on?
- What affects the angle of macroscopic magnetization rotation (immediately after the pulse, in rotating frame)?
- What happens to spin level population after irradiation of a sample by the 90º-pulse?
- What happens to macroscopic magnetization after irradiation of a sample by the 180º-pulse?
- What are the advantages of the pulse NMR as compared to continuous wave NMR?
- How would you measure a 90º-pulse?
- Which function approximates a FID bender?
- What is the name for the oscillations of a magnetization projection on a horizontal plane?
- Defocusing of which magnetization components can be removed by spin echo?
- Why homonuclear multiplets are not refocused by the spin echo?
- Which part of a complex Fourier image corresponds to the absorption signal?
- Function of which type represents a signal in the resonance coil caused by rotating magnetic moment?
- What are the mandatory attributes of a pulse program in correlation experiments?
NMR experiments
- How does the broadband decoupling of protons appear in 13Ñ spectra?
- Why decoupling is turned on before the acquisition in Gated experiments, but not during the acquisition?
- Which information is provided by the selective double resonance spectra?
- Under which circumstances the double resonance experiment is better than COSY?
- Which information is provided by COSY spectra?
- How does the width of the second pulse affect a COSY spectrum?
- How does double quantum filter affect a COSY spectrum?
- Which additional information is provided by experiments with coherence transfer, as compared to COSY?
- Which information is provided by HSQC spectra?
- How editing and H-decoupling affect an HSQC spectrum?
- Under which circumstances HETCOR is better than HSQC?
- Which information is provided by HMBC spectra?
- Under which circumstances the one-dimensional HMBC is better than two-dimensional?
- Which information is provided by TOCSY spectra?
- How can one reduce signal overlap along the diffusion axis in DOSY?
- Which information is provided by J-resolved spectra?
- Which experiments reveal a proton spin system coupled to a particular heteronucleus?
- What are the requirements to sample to run INADEQUATE experiment?
- Which experiments allow to measure heteronuclear coupling constants?
- What is the advantage of INEPT, as compared to SPI?
- What are the differences between the APT, DEPT and INEPT spectra?
- Which heteronuclear experiments with coherence transfer exist?
- Spectroscopy of which nuclei benefit the most from usage of polarization transfer and why?
- What are the advantages and disadvantages of three-dimensional experiments?
- What is the difference between HNCACO and HNCOCA?
Nuclear Overhauser effect
- Which information is provided by ROESY spectra?
- Which type of relaxation leads to NOE phenomenon?
- At which interatomic distance does NOE appear?
- What is the theoretical maximal NOE?
- How is NOE affected by the spectrometer frequency?
- What is the relationship between the phase of diagonal peaks and NOE cross-peaks in ROESY spectra?
- What is the relationship between the phase of chemical exchange cross-peaks peaks and NOE cross-peaks in NOESY spectra?
- Why are one-dimensional NOE spectra often recorded in a difference mode?
- What conditions should be met for quantitave NOE measurement?
- What is the dependance of inter-ligand NOE (in the presence of a substrate) on mixing time?
- Why does NOE observed during irradiation of water signal have different sign for binded and non-binded ligands?
- What is a content of each strip of 3D 15N-edited HSQC-NOESY?
- Which substrate concentration is required to run STD and WaterLOGSY experiments?
NMR practice
 (click here)
(click here)
Last update: 2024 Feb 12  Home
Home
